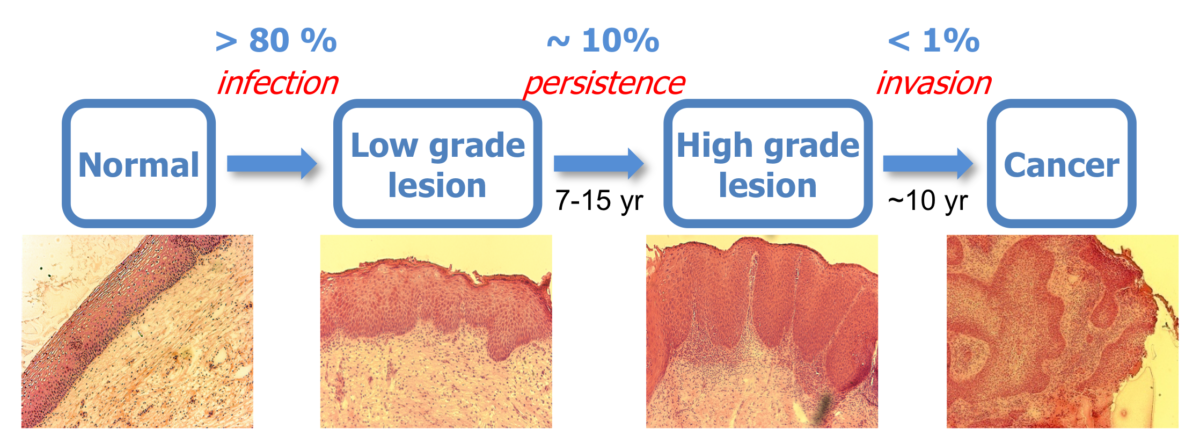

During decades of cancer progression, HPV persists, evades host surveillance, and continuously contributes to host cell transformation. However, little is known about the mechanisms of disease progression driven by HPV. To understand the molecular mechanisms that drive HPV-positive cancer development, we have analyzed global gene expression changes using hundreds of human cervical and head/neck tissues in different HPV status (HPV-positive vs. HPV-negative) and disease stages (normal, early and late precancers, and cancer). Our studies have revealed a striking cascade of distinct HPV-specific changes in several host pathways including innate/adaptive immune regulations. Particularly, the chemokine CXCL14 is significantly downregulated during HPV-driven cancer progression by HPV E7-mediated promoter methylation. Our in vivo study has shown that restoration of Cxcl14 expression dramatically suppresses tumor growth in immunocompetent syngeneic mice by recruiting natural killer and T cells. We are currently 1) investigating the detailed mechanisms of CXCL14-mediated tumor suppression and 2) developing chemokine-based novel immunotherapies to treat CxCa and HNC.